Snakes on my blog
After going to watch "Snakes on a Plane" at my local multiplex, I was inspired to do some fact-checking on the screenplay by running a quick search of the literature.
(Here come the comments about selling out on blog topics too early in my fledgling career.)
My search engine of choice was PubMed, a government service with millions of journal article abstracts searchable by topic, author, etc.
To read about the premise of this cult thriller, click here. In short, the snakes on the plane became extremely aggressive and started attacking people because of a concentrated pheromone that was sprayed onto the passengers' leis. (The plane was en route to LA from Hawaii).
Here are some of the more interesting tidbits I dug up:
the ref: Science 1989, 245, 290-3.
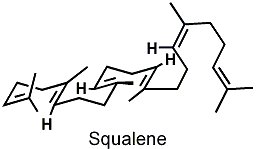
The female pheromone of the Canadian red-sided garter snake is an intricate concoction of nonvolatile saturated and monounsaturated long-chain methyl ketones, and the corresponding male pheromone contains squalene (see figure), a compound I first learned about in my chemistry classes because of its role as a key intermediate in cholesterol biosynthesis.
So, did the mafia goons just spray one chemical onto those leis, or was it an "all purpose pheromone" that would "do it" for all of about twenty or thirty different species of snakes that were wreaking havoc on the plane? The snakes were from all over the world, as the actor playing the snake expert told us numerous times. I'd guess their pheromones were slightly different, but maybe not enough for it to matter?
Another thing: It's counterintuitive to me that pheromones from the opposite sex would trigger aggression. (I know, different strokes for different folks, but still...) It would make more sense that a pheromone from a member of the same sex would get the competitive juices flowing.
A 2002 paper suggests that a certain lizard species relies mainly on scent to determine the gender of an intruder. The authors experimentally manipulated the body markings and odor of the lizards in order to learn more about the interplay between sight and smell. Among other things, they observed that male snakes responded aggressively to a snake that smelled male, even if it was painted to look like a girl.
that ref was: Aggressive Behavior 2002, 28, 154-163.
Does that mean that to minimize confusion, all the snakes on that plane had to be the same sex? Or at the very least, there couldn't be any Black Adders.
I'll stop now, because this may just be the most thought anyone's ever given to the movie after having seen it, and that'll spoil the mood.
Labels: current events