Momma said knock you out
I didn't want to miss the chance to blog about an anesthesia paper I saw in ACS Chemical Biology. :)
The ref:
ACS Chem. Biol. 2006, 1, 377-384.
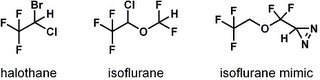
A synthetic organic chemist may scoff at the structural simplicity of anesthetics like halothane and isoflurane (see figure), but there's no doubt that the effects they have on our physiology are very complex. These compounds bring on amnesia (unconsciousness/ unawareness), analgesia (pain relief), and paralysis, and all of these effects are completely reversible. When a new anesthetic emerges, it's important to figure out the molecular mechanisms behind its activity, but researchers are still very much in the dark in this area.
It doesn't help matters that these molecules are challenging to work with in the lab. They're volatile (evaporate easily), and they don't have a very strong binding affinity for their target(s). The latter is a good thing since you want your anesthetic to wear off!
There is some data available about binding sites and targets for older anesthetics called haloalkanes (halothane is in this category), but those molecules really aren't used in human patients anymore because of their toxicity profiles. The goal of this paper is to develop a tool to get the same kind of data on the stuff we're actually using, the haloethers (like isoflurane).
To solve the low binding affinity problem, Xi and colleagues designed an isoflurane mimic (see figure) that's photoactivatable. The molecule resembles isoflurane, but it contains some chemical bonds that will break down when you shine light of a certain energy on them. Once that happens, what's left is a reactive species that they anticipated would latch onto a target, allowing them to better examine the interaction.
The conclusion of the paper was that the photoactivatable isoflurane mimic is a valid tool for research in this area. It behaves similarly to isoflurane in the petri dish and in animal tests, and binds to a known isoflurane target in a very similar place.
I did notice a couple of minor nomenclature errors in the paper: Isoflurane is 1-chloro-2,2,2-trifluoroethyl difluoroMETHYL ether, and the analogous correction also goes for desflurane.
The reactive group that the researchers engineered is called a carbene. An explanation of what that is is beyond the scope of this blog, but not of this website. Maybe someone who remembers carbene reactivity better than I do can answer the question they pose in the text: Is there an amino acid residue with which the UV-generated carbene won't react? They propose mutagenesis experiments, and I'm sure you can just buy a kit somewhere that'll take care of that, but there must be some references are already out there on this. The whole train of thought began when they didn't see the expected reactivity with tyrosine. It seems like they're trying to determine whether it's a question of intrinsic reactivity or just the molecule's "fit" in the cavity.
To do: the authors mentioned that the more old-school haloalkane anesthetics like halothane were used to fish out potential targets for interaction through photolabeling in complex mixtures. I'd like to see that experiment done with this analog. This kind of experiment has already been done in many other systems (for example, see here). I guess it just depends on how easily they can incorporate a visualization element.
Labels: literature


0 Comments:
Post a Comment
<< Home